Abstract
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is an ultra-rare acquired genetic disease characterized by chronic intravascular hemolysis due to uncontrolled complement activation. Despite available treatments, patients may continue to experience episodes of breakthrough hemolysis. Cemdisiran and pozelimab are investigational treatments that act together to suppress terminal complement activity. Cemdisiran is an N-acetylgalactosamine-conjugated small interfering RNA (siRNA) that suppresses liver production of complement component C5, while pozelimab is a fully human monoclonal antibody inhibitor of C5. The combination of pozelimab and cemdisiran is being evaluated in an ongoing phase 2, randomized, open-label, two-arm study (NCT04811716) that is designed to assess the safety and efficacy of the combination in patients with PNH who have transitioned from pozelimab monotherapy. The interim safety and efficacy results are presented here.
Methods: Patients (n=22) were randomized (1:1) into one of two treatment arms; both arms received subcutaneous (SC) cemdisiran 200 mg every 4 weeks (Q4W) plus pozelimab 400 mg SC at a frequency of either Q4W (arm 1) or every 2 weeks (Q2W; arm 2). The study consists of four periods: a screening period (7-8 days), an open-label treatment period (OLTP; 28 weeks), an optional open-label extension period (OLEP; 52 weeks), and a safety follow-up period (52 weeks).
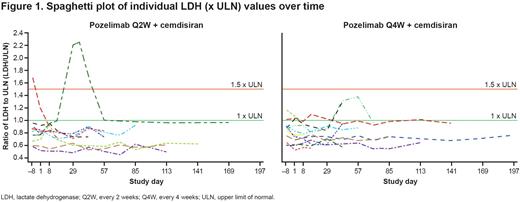
Results: At the time of this interim analysis, all 22 randomized patients were ongoing in the study (median duration of 57 days), including one who had completed the OLTP and was continuing in the OLEP. Twenty-one patients (95.5%) maintained control of lactate dehydrogenase (LDH; ≤1.5 x upper limit of normal [ULN]) up until the data cut-off (Figure 1). In addition, most patients maintained an LDH value below 1.0 x ULN for most of the observation period. At the time of data cut-off (March 18, 2022), 90.9% of patients (n=20) met the criteria for hemoglobin stabilization (did not receive a red blood cell transfusion, no decrease in hemoglobin level ≥2 g/dL). One patient with breakthrough hemolysis (in association with a chlamydia infection) had a decrease in hemoglobin level ≥2 g/dL and had a transfusion, while another patient had a transfusion only. CH50, a measure of total complement hemolysis activity, remained fully suppressed at all post-baseline time-points measured, including during the aforementioned breakthrough hemolysis event. There were no serious treatment-emergent adverse events (TEAEs) or TEAEs leading to study drug discontinuation in any patient from either treatment group. Importantly there were no meningococcal infections or TEAEs leading to death in this study. Six patients (27.3%; three from each treatment arm) experienced a total of 12 TEAEs. One patient (8.3%) in arm 1 experienced one adverse event of special interest, a mild injection-site reaction characterized by stinging lasting 30 minutes. All TEAEs were of mild-to-moderate intensity, except for a single severe TEAE of anemia occurring in one patient from the pozelimab Q2W + cemdisiran treatment group (arm 2). This same patient had previously experienced a moderate non-serious TEAE of breakthrough hemolysis, which was not considered related to the study treatment by the investigator.
Conclusions: The combination of pozelimab and cemdisiran was generally well tolerated in patients with PNH who transitioned from pozelimab monotherapy (NCT04162470), regardless of treatment arm. Over 90% of patients maintained adequate control of hemolysis and achieved hemoglobin stabilization. Furthermore, most patients maintained normalization of their LDH for most of the observation period. These findings support the ongoing development of this combination therapy in the treatment of patients with PNH.
Disclosures
Wong:F. Hoffmann-La Roche AG: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis Pharmaceuticals: Research Funding, Speakers Bureau. Pavani:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Aurand:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Dain:Regeneron Pharmaceuticals, Inc.: Current Employment. Perlee:Regeneron: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Souttou:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Weyne:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kelly:Swedish Orphan Biovitrum AB: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: educational work sponsored by Apellis with unrestricted grant paid to Medscape; Biocryst: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: conference support; Sobi: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Biologix: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal